TEDA-made Rocket Heart Approved
Developed by the TEDA-based Rocketheart Technology Co., Ltd, Rocket Heart-HeartCon, Implantable left ventricular assist device, was approved for marketing. As an implantable magnetic-hydrodynamic suspension left ventricular assist device, HeartCon is the first domestic originally-developed product with independent intellectual property rights. It is the only product made by a purely state-owned enterprise, the only combination of medicine and engineering from beginning to end and the only product developed and made in China, with the only domestic original independent intellectual property rights. Its key technical indicators meet the world’s advanced standards. This artificial heart will end the helplessness when treating the end-stage heart failure in China and bring benefits to patients and their families.
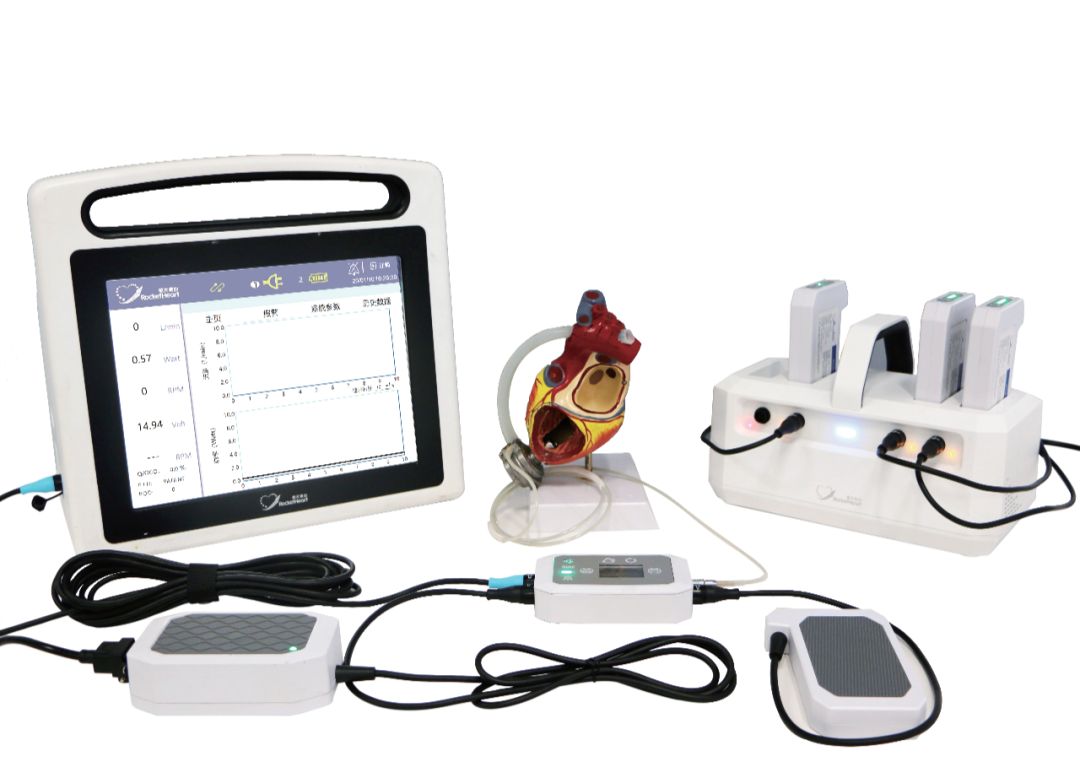
Founded in 2016, Rocketheart Technology Co., Ltd. was jointly founded by TEDA State-owned Assets Management Company and China Academy of Launch Vehicle Technology. Its artificial heart developed has been listed as a medical device to be developed in the 14th five year special plan of Tianjin’s bio-pharmaceutical industry.












 津公网安备 12019002000128号
津公网安备 12019002000128号

