TEDA-made Rocket Heart to Hit the Market in 2022
“Rocket Heart” HeartCon, the first artificial heart developed by the TEDA-based Rocketheart Technology Co., Ltd. passed on-site inspection recently. The artificial heart started clinic trials on September 15th, 2020, and received acceptance for product registration in December 2021. It is scheduled to go on sale this year.
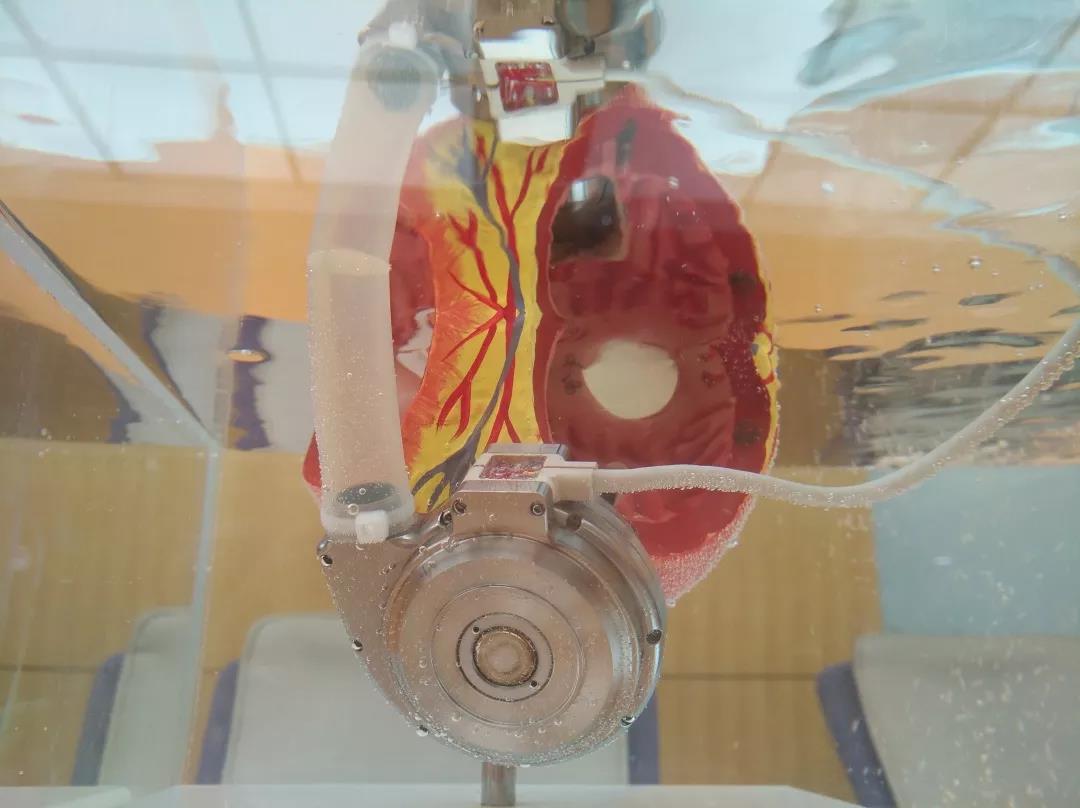
As a 3rd-generation implantable magnetic-hydrodynamic suspension ventricular assist device, HeartCon is a domestic originally-developed product with independent intellectual property rights with its main indicators meeting the world’s advanced standard. Tianjin TEDA International Cardiovascular Hospital, the main researcher of HeartCon, works with a dozen of domestically leading hospitals in cardiac surgery or heart diseases, creates China’s first clinic trial cooperation of multi-center artificial heart, and becomes the first unit applying the clinic trial in China.
Rocketheart Technology Co., Ltd., jointly founded by TEDA State-owned Assets Management Company, China Academy of Launch Vehicle Technology and Beijng Research Institute of Precision Electromechanical Controlling Devices, is committed to being the supplier of lifecycle heart failure products. With the unswerving adherence to the path of civil-military integration and combination of medicine and engineering, the company sets up Tianjin Artificial Heart Key Laboratory of Enterprise, Tsinghua University-Rocketheart Artificial Heart Joint Laboratory, Academician Workstation, etc. So far, its second artificial heart with half the weight of HeartCon, is able to provide treatment solution for more patients with heart failure.












 津公网安备 12019002000128号
津公网安备 12019002000128号

