China's First! Another TEDA-Developed Product Obtains International Certification
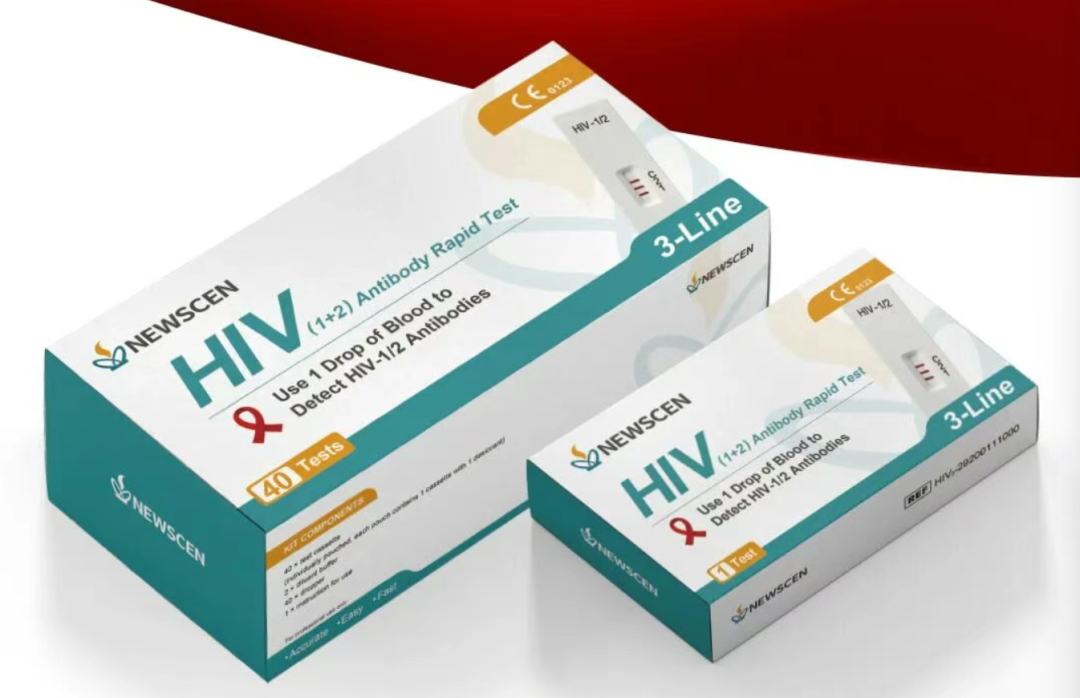
Recently, Tianjin NewScen Coast Bio-Pharmaceutical Co., Ltd., a company based in TEDA, announced that its independently developed HIV (1+2) three-line antibody rapid detection kit has been certified under the European Union's In Vitro Diagnostic Medical Devices Regulation (IVDR). This makes it China's first HIV three-line product to receive IVDR certification at the highest risk class. This achievement is not only a significant milestone in NewScen Coast Bio-Pharmaceutical's development but also a crucial step for China's in vitro diagnostics (IVD) industry towards high-end and international development.
NewScen Coast Bio-Pharmaceutical's HIV (1+2) product demonstrates exceptional performance. According to test results from Qarad B.V.B.A. in Belgium, an EU-designated laboratory, the product can detect antibodies as early as 7 days after infection. Currently, no other similar products are able to detect antibodies earlier than this window period, and the positive detection capability is significantly better than that of similar rapid test products. Performance evaluation data from the World Health Organization shows 100% sensitivity and specificity. Furthermore, IVDR clinical evaluation results indicate 100% sensitivity and 99.82% specificity, strongly confirming its superior performance in early diagnosis and diagnostic accuracy.

NewScen Coast Bio-Pharmaceutical Co., Ltd. was established in TEDA in May 2003 and listed on the National Equities Exchange and Quotations (NEEQ, also known as the "New Third Board") in December 2017. It is one of China's early POCT (Point-of-Care Testing) manufacturers and a National High-tech Enterprise. The company primarily focuses on technological innovation and industrial upgrading in the field of in vitro diagnostic rapid testing. Its products cover various fields, including cardiovascular and cerebrovascular diseases, infectious diseases, inflammation, hormones, kidney injury, and tumors.











 BACK TO THE PREVIOUS PAGE
BACK TO THE PREVIOUS PAGE

 津公网安备 12019002000128号
津公网安备 12019002000128号

